
TITAN Award Announcement
October 14, 2025 – Hologenix, LLC, the inventors of CELLIANT® infrared technology, is proud to announce that we have been honored as a Gold Winner in the 2025 TITAN Innovation Awards in the category of…
Part of our mission is to impact the most people possible with the benefits of infrared. From improved quality of life, to better performance, faster recovery, and more restful sleep, we believe there are endless communities around the world who can benefit from CELLIANT®.
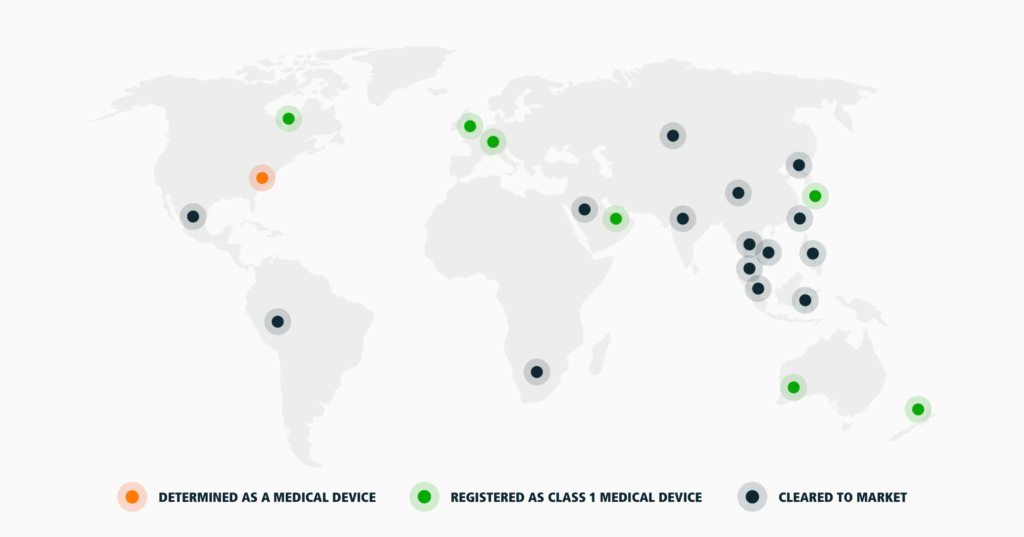
That’s why we are so proud to announce that our regulatory status now reaches over 50 countries, making CELLIANT an ideal global partner.
As our co-founder and CEO, Seth Casden puts it, “We’ve laid the groundwork for our partner brands to capitalize on the benefits of our infrared technology and to enhance their ability to do business. We firmly believe that regulatory status matters and that is why we have grown the number of countries we have such relationships with by over a third in the last three years. It is definitely a competitive advantage of our company and CELLIANT.”
Our growth in regulatory status is also an indication of our commitment to science. We have a Science Advisory Board composed of experts in the fields of photobiology, nanotechnology, sleep medicine, diabetes and wound care that have overseen 9 peer-reviewed published studies and our rigorous testing methods, collectively demonstrating CELLIANT’s effectiveness and the benefits of infrared energy. These studies form the basis of our ability to partner with global regulatory bodies to allow our brand partners to make infrared (IR) benefit claims.
CELLIANT has recently celebrated our 20th anniversary and the growth in regulatory status has been steady and in step with the growth of our team globally. We now have offices on 4 continents with 9 global sales support employees and 10 different legal and regulatory firms. CELLIANT is determined or designated as a Class 1 medical device in 38 countries and cleared to market in a further 15 countries.
Our global growth has led to some great products with brand partners around the world, including:

CELLIANT is determined or designated as a Class 1 medical device in 38 countries:
CELLIANT is cleared to market in 15 countries:
For our purposes, cleared to market means that, based on research in a specific country or region, the evidence supports a conclusion that CELLIANT products and the proposed claims fall outside of the definition of a medical device and no medical device registration is required. In other words, independent assessments have been made that permit certain CELLIANT claims to be made as a non-medical device.
If you’d like to learn more about incorporating CELLIANT into your textile products and how they can help differentiate your brand, please fill out the form below – we would love to hear from you.



October 14, 2025 – Hologenix, LLC, the inventors of CELLIANT® infrared technology, is proud to announce that we have been honored as a Gold Winner in the 2025 TITAN Innovation Awards in the category of…

October 3rd, 2025 – This year, CELLIANT is thrilled to be part of LongevityFest 2025, the premier event on health optimization and longevity medicine, offering excitement on-site in Booth #12062…

September 05, 2025 – Join CELLIANT at Booth G20 in Hall A1 at this fall’s Performance Days trade show to explore how CELLIANT helps transform everyday apparel into passive wellness tools…
To access all of our reports please input your email below.
Contact us at marketing@celliant.com
Thank you for your request. Please download the brochure below.
Sign up for the latest Celliant news and innovations.
REQUEST INFORMATION