
Introducing CELLIANT XRF Fabric Testing
May 01, 2025 – The pursuit of continual improvement has led us to adopt a powerful new method of analysis for CELLIANT-powered fabrics: X-Ray Fluorescence (XRF) Testing…

Testing CELLIANT® through clinical trials designed by our Science Advisory Board is critical to our commitment to transparency and demonstrating CELLIANT’s efficacy. We’re very proud to announce our 10th published study, now published in Journal of Textile Science & Engineering and titled, Randomized Placebo-Controlled Clinical Trial of Gloves and Stockings Made from Infrared-Emitting Fabric (Celliant®) for Transcutaneous Oxygen and Peripheral Blood Flow in Diabetic Patients with Vascular Impairment.
As the conclusion in the study states:
The trends that were observed in favor of CELLIANT stockings suggest that a larger well-designed clinical trial should be undertaken, and may provide evidence of clinical efficacy in treatment of the diabetic foot.
We encourage anyone in the field of diabetic research and treatment, who is also interested in the potential for infrared textiles to help people with diabetes, to get in touch with our team via the form below and discuss our hypotheses, outcomes and research protocols.


May 01, 2025 – The pursuit of continual improvement has led us to adopt a powerful new method of analysis for CELLIANT-powered fabrics: X-Ray Fluorescence (XRF) Testing…
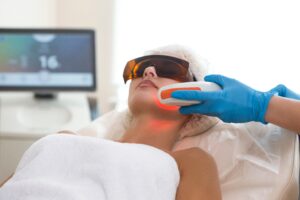
February 21, 2025 – Photobiomodulation (PBM), sometimes referred to as low-level light therapy, is a form of light therapy that uses specific wavelengths of red and near-infrared…

September 13, 2024 – Understanding metabolic rate is important because it plays a key role in energy levels and overall well-being. While metabolic rate varies…
To access all of our reports please input your email below.
Contact us at marketing@celliant.com
Thank you for your request. Please download the brochure below.
Sign up for the latest Celliant news and innovations.
REQUEST INFORMATION