Is CELLIANT Right for You? A Use Case Breakdown by Lifestyle
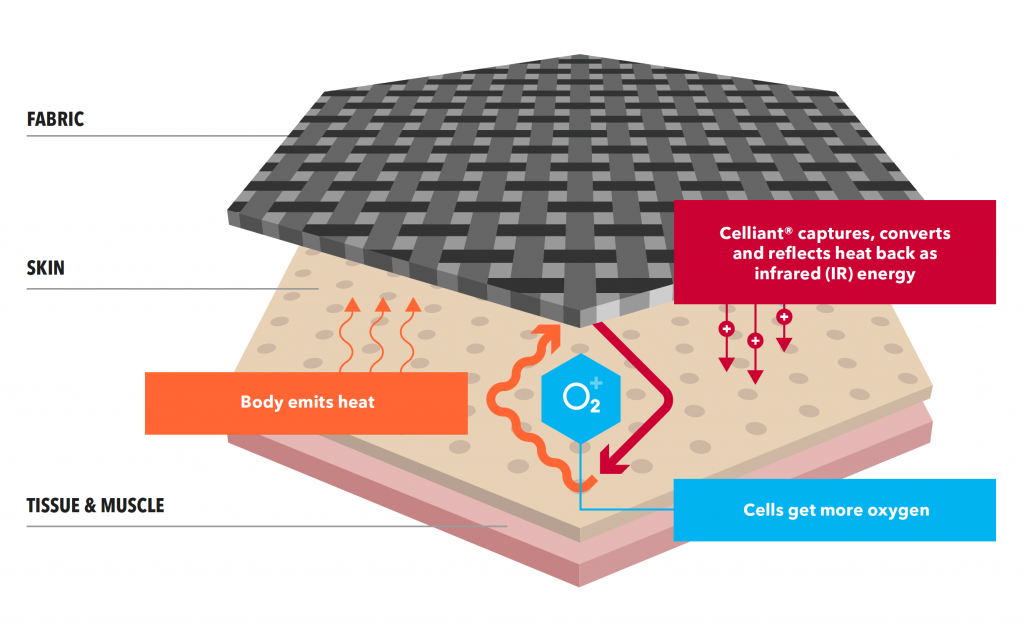
July 10, 2025 – In a growing world of performance and wellness textiles, CELLIANT stands out as a clinically tested tech that transforms body heat into infrared energy. But how do you know if CELLIANT is right for you?