
Introducing CELLIANT XRF Fabric Testing
May 01, 2025 – The pursuit of continual improvement has led us to adopt a powerful new method of analysis for CELLIANT-powered fabrics: X-Ray Fluorescence (XRF) Testing…




May 01, 2025 – The pursuit of continual improvement has led us to adopt a powerful new method of analysis for CELLIANT-powered fabrics: X-Ray Fluorescence (XRF) Testing…
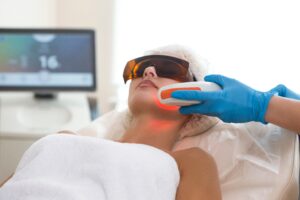
February 21, 2025 – Photobiomodulation (PBM), sometimes referred to as low-level light therapy, is a form of light therapy that uses specific wavelengths of red and near-infrared…

September 13, 2024 – Understanding metabolic rate is important because it plays a key role in energy levels and overall well-being. While metabolic rate varies…
To access all of our reports please input your email below.
Contact us at marketing@celliant.com
Thank you for your request. Please download the brochure below.
Sign up for the latest Celliant news and innovations.
REQUEST INFORMATION