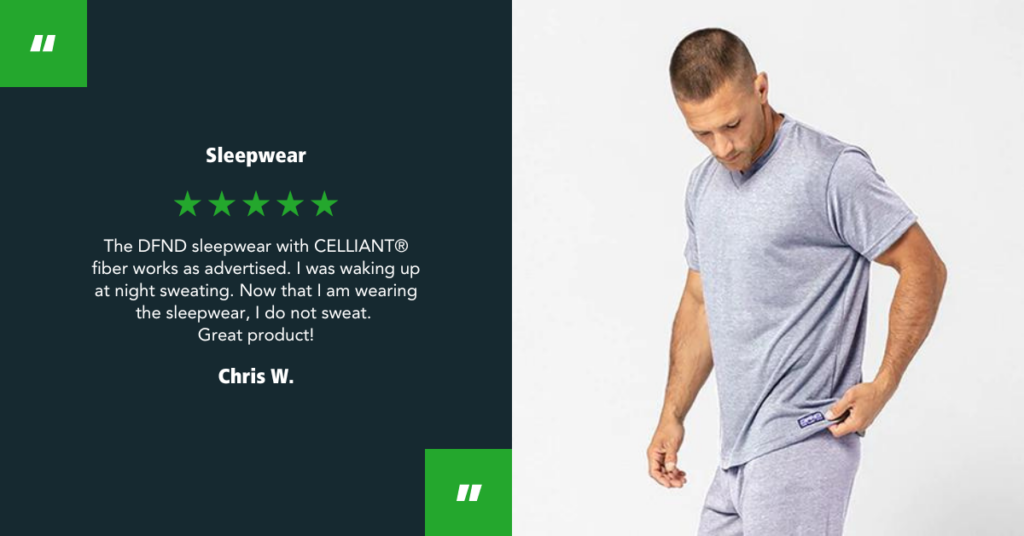
Kilde Medic x CELLIANT Partnership
May 9, 2025 – Kilde Medic and CELLIANT® have been partnered since 2022, united in their shared goal to deliver infrared wellness to people seeking to support their overall wellbeing. Through their brand, Reflexwear, Kilde Medic has created a standout line…